
The vast amounts of genomic data being collected and analyzed today provide the basis for our current knowledge of human genetics and many future developments in personalized medicine.
The amount of genomic data is growing exponentially as advances in technology enable rapid, inexpensive whole-genome sequencing of individuals. With this explosion of genomic data comes the opportunity to develop a greater understanding of human biology than ever before.
Genomic data is significant because of its potential to enable personalized medicine. Personalized medicine uses an individual’s unique genomic data to determine appropriate treatment options based on that individual’s specific genetic profile. Using an individual’s genomic data allows for a much more tailored approach to treatment than the traditional one-size-fits-all approach used in many medical practices today.
An example of how genomic data can be utilized to improve an individual’s care is through pharmacogenomics. Pharmacogenomics analyzes how an individual’s genetic profile affects their response to different medications, which enables their physician to prescribe the most appropriate medication with the fewest adverse side effects.
Pharmacogenomics has greatly improved cancer treatment by providing physicians with the genetic information needed to design more effective treatment plans tailored to each patient’s tumor.
In addition to identifying genetic causes of many diseases, the data will also allow scientists to determine whether there are genetic tendencies toward disease. By analyzing genomic data from the entire population, researchers will identify genetic markers that indicate an individual is at higher risk of diseases such as diabetes, heart disease, and cancer.
Genomic Data Analysis is very important to preventive health care because, using genetic information as a guide, people can choose lifestyles and undergo medical tests (screenings) to detect diseases they are genetically predisposed to develop.
Genomic Data Analysis, although very powerful, has its own challenges. The amount of genomic data being produced requires sophisticated data analysis tools (driven by AI and Machine Learning) to sort through all the data and identify relationships or correlations that would be difficult or impossible to find with non-AI/Machine Learning-based analytical techniques.
As genomic data becomes more accessible, other privacy and ownership issues arise. For example, when individuals agree to let their genomic data be analyzed, the question of who will control the data and how it will be used arises.
Genomic data is one of the most important tools that modern medicine can utilize to provide us with an unparalleled look into our health and diseases. The opportunity to personalize medicine for individuals based on their genetic code, the ability to identify whether we have a higher risk of contracting a disease based on our genetics, and the ability to make informed health care decisions are just a few examples of how genomic data is revolutionizing the medical industry.
Genomics will be at the forefront of the medical industry for years to come, and it will also lead the way in creating the next generation of medicine.
Have you ever sat in a doctor’s office waiting for a diagnosis? Medicine has traditionally used symptoms and a patient’s family history to diagnose a condition. However, what if your doctor had access to a ‘blueprint’ of your body, your own personal genetic code, and a highly intelligent computer system to help your doctor identify potential problems with your health before they occur?
This is no longer science fiction; this is becoming a reality as genomic data is being combined with Artificial Intelligence (AI).
The most significant issue with all biological blueprints has always been size. A person’s entire genetic map (or genome) includes over 3 billion letters. According to the National Human Genome Research Institute, if someone were to print out an individual’s entire genome, the stack of paper would reach a height of 200 feet.
Therefore, no group of scientists could possibly go through such a large amount of information to identify the small, relevant differences in genetic maps.
AI in health care provides a solution by allowing us to train AI to read very large amounts of genomic data. We can simultaneously review thousands of large genomic libraries using AI to identify patterns associated with disease states, such as cancer or Alzheimer’s. The combination of genomic data and AI enables us to move from responding to illness to preventing illness.
What Is a Genome, and Why Is It More Than Just Your Genes?
Most people associate their DNA with genes — the instructions for specific characteristics such as eye color, hair color, or height. To make it easier to visualize a gene, think of a gene as a single recipe in an extremely large cookbook. A gene would be responsible for the recipe to create the pigments in your eyes.
Another gene would provide the recipe for how your body metabolizes sugar. While important, genes represent only a portion of a much larger story.
If we continue this analogy and consider a gene as only one recipe in the cookbook, then your genome represents all the books in the cookbook — essentially all the instructions required to build and operate you.
Your genome is composed of approximately three billion letters written in a very simple four-letter alphabet (A, C, G & T). The order in which these letters appear is what makes you unique.
In addition to determining your physical characteristics, your genome contains information about your potential disease risks and how your body may respond to medication. As such, reading a library of this size is an enormous task.
The Ultimate ‘Big Data’ Problem: Why Can’t Humans Just Read the Genome?
In a surprising twist, the biggest problem is not in reading an individual’s genetic code; it is in comparing the genetic codes of thousands or millions of individuals side-by-side to identify the small number of genetic variations relevant to our health.
At this point, the task shifts from being very difficult to being fundamentally impossible for a human to accomplish.
Consider the magnitude of the comparison in basic terms as follows:
- Spotting a typo between two sentences? A task easy for most anyone.
- Identifying a different word among the pages of two books? Difficult, but feasible with sufficient time.
- The identification of a single, unique spelling error found across all of the libraries (including the New York Public Library) containing over 10,000 different book collections represents the true scope of contemporary genomic analysis.
Scientists are searching for subtle patterns (i.e., slight deviations from the standard genetic code) that occur in individuals with Alzheimer’s disease (and other conditions) but not in those without. The scientists’ large-scale comparisons are what will help them unlock the mysteries of diseases.

This is the ultimate “big data” problem. For many years, the monumental task of comparing all of these “books” was the rate-limiting step; therefore, researchers were unable to convert their scientific discoveries into medical treatments as quickly as they would have liked.
No matter how intelligent or capable a researcher is, he/she cannot quickly compare the vast amounts of information in genomics. Therefore, we need a new tool to assist us in comparing all the “books” across all the libraries simultaneously and identifying patterns that we cannot identify ourselves.
AI in Genomics: Transforming How We Understand DNA

“A.I. in Genomics: A New Paradigm for Analyzing Genomic Data with Artificial Intelligence” is an innovative approach that leverages artificial intelligence to analyze large-scale genomic data. AI in genomics enables researchers to analyze massive datasets of genetic information and identify patterns beyond human capability.
The paradigm shift in how we understand DNA not only accelerates research but also provides researchers with deeper insight into the complexity of biological systems.
The incorporation of AI into genomics enables the identification of key genetic markers associated with disease states. AI algorithms can identify subtle differences in genomic data across populations that increase the likelihood of developing specific diseases.
This type of capability has significant implications for personalized medicine, where AI in genomics can help develop treatment options tailored to an individual’s genetic profile.
The use of AI in genomic research directly affects the speed at which new drugs can be developed. The traditional method of drug discovery takes a long time and is very expensive, often taking many years before a result is produced. Using AI, scientists can run simulations of thousands of drug interactions using genomic information, potentially shortening the drug discovery timeframe from years to weeks.
In addition to increased efficiency, this allows researchers to develop potential treatments for genetic disorders (diseases caused by a defect in a single gene) that would otherwise receive little to no attention due to the limited number of patients.
AI in genomics also provides an important tool for predictive analytics. By analyzing genomic data, AI models can predict a patient’s risk of developing specific diseases. Additionally, by predicting a patient’s risk of developing certain diseases, it empowers the patient to take proactive steps to manage their health, a critical step toward moving away from a purely reactive medical model toward one of prevention.
To summarize, the combination of genomic data and AI is changing how we understand DNA and is leading the charge toward personalized medicine, speeding up drug development, and encouraging a proactive health model. The future of genomics is certainly promising, thanks to advances enabled by AI.
How AI Becomes a ‘Super-Powered Pattern Finder’ for Our Genes
This is the point at which AI can help identify patterns in DNA data by finding the most likely patterns, not by thinking or being conscious, but by maximizing likelihood. To explain how AI finds patterns in DNA data, consider a spell-checker that can search for errors much faster than you can read a word.
The spell-checker does not have to understand the content of your writing to identify a spelling error; it just has to recognize how the spelling should be and look for the errors within seconds. Likewise, AI can review billions of DNA letters from thousands of people to identify the small, consistent changes that humans cannot see.
Scientists have developed a method to do this, which involves a process that is very much like that used to teach an individual to recognize various animals. In doing so, they don’t provide the AI with complex biological rules. Rather than this, they “teach” the AI by providing it with many thousands of examples.
For example, scientists may provide the AI with the genomes of 50,000 individuals with a specific type of cancer and 50,000 individuals without it. The purpose of the AI is not to develop a comprehensive understanding of cancer.
Its task is to analyze each set of data provided to determine if there exist in the first group (the individuals with cancer) unique genetic signatures (unique patterns of genetics), that are consistently present in that group but are not identified within the second group (the individuals that do not have cancer).
Once the AI has been able to identify at least one of the key genetic signatures(s), the scientist has identified the biomarker; think of a biomarker as a biological signpost (a “check engine” light for the human body), written into our DNA.
A signpost such as this can indicate a greater risk of developing a particular disease, predict how an individual will respond to a drug, or even identify the underlying cause of the tumor. Identifying these biomarkers is the first step towards treating individuals based on their unique genetic characteristics rather than a “one size fits all” approach.
AI Genomics: Where Biology Meets Intelligent Systems

Innovative AI Genomics – “Where Intelligent Systems Meet Biological Data”- is a new field where the power of Artificial Intelligence meets the complexity of biology. Researchers utilizing AI Genomics are changing the way that genomic data is analyzed and interpreted, creating a pathway for significant advancements in healthcare and disease management. The fusion of intelligent systems with large volumes of biological data has enabled us to quickly and efficiently process genomic data.
The use of AI Genomics to analyze genomic data across multiple populations creates the opportunity to discover new relationships within biological processes. Machine learning can be applied through AI Genomics, allowing researchers to establish connections between specific genetic variants and disease, thereby increasing our understanding of disease complexity (e.g., cancer and diabetes). A better understanding of each patient’s genetic makeup will create opportunities to develop targeted therapeutic interventions tailored to their individual genetic profile.
Beyond that, AI Genomics enables researchers to accelerate their work by increasing the speed at which they can interpret large amounts of data. Analyzing genomic data in a traditional manner can be extremely time-consuming and create bottlenecks in research progress. AI-based tools can automate many of the traditionally laborious aspects of this process, allowing researchers to analyze data and derive meaningful conclusions without spending excessive time simply performing the analysis.
AI Genomics has a wide variety of applications as well. From helping scientists identify better drugs to improving diagnostic accuracy, AI Genomics is revolutionizing the practice of personalized medicine. In addition, using genomic data more effectively will enable health care providers to offer patients more tailored treatment options based on their unique genetic makeup, ultimately achieving better outcomes.
To summarize, AI Genomics is positioned at the forefront of modern biological research in the use of genomic data through intelligent systems. The integration of these two technologies is expanding our knowledge of biology and enabling us to begin envisioning how we may improve the delivery of health care in the future through increased personalization and efficiency. The growth of AI Genomics will continue to open numerous opportunities to better understand life at the genomic level.
AI Data Analysis: Making Sense of Massive Genomic Datasets

“AI Data Analysis: Making Sense of the Mountains of Genomic Data” is a major step forward in the field of genomics, where both opportunities and challenges arise in analyzing large amounts of genomic data.
The evolving nature of genomic sequencing technologies creates an increased need to understand how to analyze and interpret this vast amount of data. Therefore, AI data analysis is emerging as a tool for genomic data analysis, using machine learning algorithms to rapidly sort through and accurately identify complex genomic information.
The application of AI to genomic data has the potential to enable researchers to identify associations between specific genetic variations and disease, thereby enabling breakthroughs in personalized medicine. By leveraging AI for genomic data analysis, researchers can explore the meaning of the data they collect rather than being overwhelmed by its sheer volume.
“Moreover, AI data analysis facilitates an integration of different types of genomic data, i.e., gene expression profiles and SNP data. A comprehensive consideration of all aspects of genomic data will be essential to fully understand the biological pathways behind health disorders. Through AI data analysis, new biomarkers can be identified, which are crucial for early detection and treatment strategies for diseases.
Additionally, AI data analysis enables faster drug discovery by rapidly analyzing genomic data. Genomic data will be analyzed to identify possible therapeutic targets and facilitate the rapid development of new treatments. Rapid discovery cycles for identifying therapeutic targets and developing drugs are particularly important in the treatment of rare diseases, since early results can have a significant impact on patients’ lives.
In conclusion, AI data analysis is a revolutionary technology to make sense of large amounts of genomic data. Through the use of AI as an intelligent system to interpret genomic data, researchers can uncover vast amounts of knowledge about biology and create new opportunities to develop more effective and individualized medical treatments.”
Genomic Machine Learning: Teaching AI to Learn from DNA

The application of advanced machine learning techniques to genomic data is a growing field known as Genomic Machine Learning (GML). GML provides researchers with a means to effectively analyze and interpret large-scale genomic datasets by integrating genomics and machine learning.
GML utilizes complex algorithms to find patterns and correlations in genomic data. By training artificial intelligence (AI) models on diverse datasets, researchers can discover significant genetic markers associated with specific health conditions.
The use of Genomic Machine Learning has both accelerated discovery and increased the precision of predictive models for disease susceptibility and treatment response.
One major advantage of Genomic Machine Learning is its capacity to rapidly process and analyze large amounts of genomic data. Most traditional analytic methodologies have been unable to efficiently manage the amount of data generated by current high-throughput technologies, resulting in bottlenecks to progress.
However, Genomic Machine Learning has streamlined this process, enabling researchers to derive actionable insights from their study results.
The use of genomic machine learning has opened up the world of personalized medicine. Genomic data will be used by healthcare professionals to create tailored treatments for each patient that are based on that patient’s unique genetic profile.
The move away from a “one size fits all” approach to using personalized medicine is truly a major breakthrough in medicine.
The uses of genomic machine learning also go beyond medicine. It has the potential to make a difference in agriculture, biotechnology, and evolutionary biology as well, demonstrating its versatility and power.
Genomic machine learning is changing how we train A.I. to learn from DNA. Using genomic data analysis to gain insight into the future of genomics, create new avenues for research, improve health outcomes, and develop treatment options for the future of science is possible with machine learning technologies.
Genomic Insights: Unlocking Hidden Patterns in DNA

“Unlocking Hidden Patterns in DNA through Genomic Insights: A New Paradigm to Understanding Human Biology and Disease” is a new paradigm to unlock the complexity of human biology and disease.
Advanced statistical and analytical methods allow investigators to mine vast amounts of genomic data to identify hidden patterns within an individual’s genome, which can contribute to health and disease outcomes. The study of genomic data is becoming increasingly important for the development of personalized medicine and for improving understanding of biological processes.
The analysis of genomic data enables investigators to develop insights and identify genetic variants associated with various health conditions by analyzing large populations to determine whether there is a correlation between genetic markers and disease susceptibility; this has led to major breakthroughs in genetics and pharmacogenomics.
These findings have allowed investigators to develop predictive models of disease risk and to provide evidence-based guidance for the delivery of targeted treatments to high-risk individuals, thus improving the efficiency and effectiveness of healthcare delivery.
Genomic insights, by enabling predictive models to forecast disease risk based on individuals’ genetic information, also have the potential to provide a clearer picture of how genetic predisposition interacts with environmental influences, thereby creating a much broader view of health.
Genomic insights are applicable beyond human health and will likely be very important in agriculture and conservation biology as well. For example, by understanding the genetic makeup of endangered crops and species, scientists can develop sustainable management practices to preserve biodiversity.
The combination of artificial intelligence (AI) and machine learning with genomic data will enable faster, more effective identification of genomic insights from large datasets. These technologies will enable researchers to rapidly analyze large datasets to identify key genomic insights that could potentially lead to new treatments and interventions.
Overall, genomic insights are revealing new patterns in DNA and providing valuable information to shape the future of medicine and biological research.
The ability to more accurately and effectively analyze genomic data will allow us to gain a greater understanding of genetics and ultimately enhance our abilities to predict, prevent, and treat many different types of health conditions. Ultimately, the potential of genomic insights is vast and represents a new scientific and medical paradigm.
Genomic Analytics: Turning Genetic Data into Actionable Knowledge

Genomic analytics aims to transform uninterpreted genomic data from an individual or population into actionable knowledge that guides healthcare providers and/or researchers in their decision-making.
By using highly sophisticated algorithms and statistical models (computational methodologies), genomics analytics enables researchers and clinicians to translate large amounts of complex genetic information into usable knowledge. The end result of this process is that genomics analytics is the foundation for many of the future advances in personalized medicine and our overall understanding of diseases.
Genomic analytics uses a systematic approach to interpret and analyze genomic data generated by experiments, including but not limited to DNA sequencing, RNA expression measurements, and epigenetic modification measurements.
These types of experiments provide a wealth of information about how biological systems function and how disease progression occurs. Genomic analytics can be used to identify patterns and relationships in data generated by these experiments that would be difficult to detect by other means.
Another advantage of genomic analytics is its potential to identify genetic variants that may increase an individual’s susceptibility to certain health problems.
Researchers have demonstrated that they can correlate genetic markers identified in genomic data with clinical outcomes to build predictive models that identify those most likely to be at risk of developing various health problems. Identifying those at risk enables healthcare providers to develop targeted preventive strategies and tailor treatment plans to meet the unique needs of each patient, ultimately resulting in better health outcomes.
In addition to its use in human health, genomics has many applications in other areas, such as agriculture and evolutionary biology. In agriculture, genomic analysis could be used to improve crop breeding programs by identifying traits related to crop yields and resilience.
Additionally, genomic analysis may help conservationists assess the level of genetic diversity within species at risk of extinction, providing insights that can inform conservation methods.
Genomic Analysis provides a critical link from the conversion of genomic data into actionable knowledge. Genomic Analysis enables scientists to analyze and interpret large amounts of genetic data, providing insights that can lead to improved health outcomes and new innovations across a variety of disciplines.
Genomic Analytics is becoming increasingly important and will continue to be so as it evolves, transforming our understanding of genetics and its impact on humans.
From Guesswork to Guarantee: How AI Enables Personalized Medicine
In addition to identifying these genetic markers that lead to a diagnosis, medicine becomes incredibly accurate. For years, treatments have been somewhat generic – like a mechanic using the same three tools on all cars, regardless of make or model.
Precision Medicine enables doctors to treat an individual with an illness rather than a group of people with similar symptoms. It’s much like the difference between buying a suit off the rack versus having a custom-made suit made to fit your body exactly.
Precision medicine is only possible because of AI’s ability to process information. After a biomarker is found in your genome, an AI system is able to reference it against massive databases of medical information.
Machine learning in genomics also allows a physician to ask a new and powerful question: “Which medication has shown the greatest efficacy and the least amount of side effects for someone with a specific genetic signature?” The AI is essentially a genius researcher’s assistant, comparing thousands of past cases to determine which treatment is most likely to yield the greatest benefit for you.
The largest influence will be on Cancer Treatment. Chemotherapy has always been an “all or nothing” approach, destroying all types of cells (cancer and non-cancer).
Today, using Artificial Intelligence (AI) to develop Personalized Medicine allows for the analysis of a tumor’s individual genetic makeup to identify its specific weakness – its “Achilles Heel”. With this information, doctors can utilize Targeted Therapy – a new family of “smart drugs” specifically developed to destroy the identified weakness and only the weakness; Thus allowing for a much more effective treatment while significantly reducing damage to the rest of the body.
Spotting the Shadow: Using AI to Predict Genetic Diseases Years in Advance
The potential for predictive medicine, therefore, lies in developing AI capable of identifying the signs of a disease before they manifest. Because many chronic diseases (e.g., Alzheimer’s and cardiovascular disease) do not have one “disease gene” but rather multiple genes working together in concert to increase the likelihood of a disease in the future; by comparing millions of genomes,
AI will be able to develop algorithms to detect combinations of low-level genetic variations that will indicate an increased risk of future disease. In essence, AI’s ability to predict genetic diseases represents a biological early warning system (i.e., an AI-powered biological smoke detector) that would alert clinicians to potential problems well before the disease develops into a clinical condition.
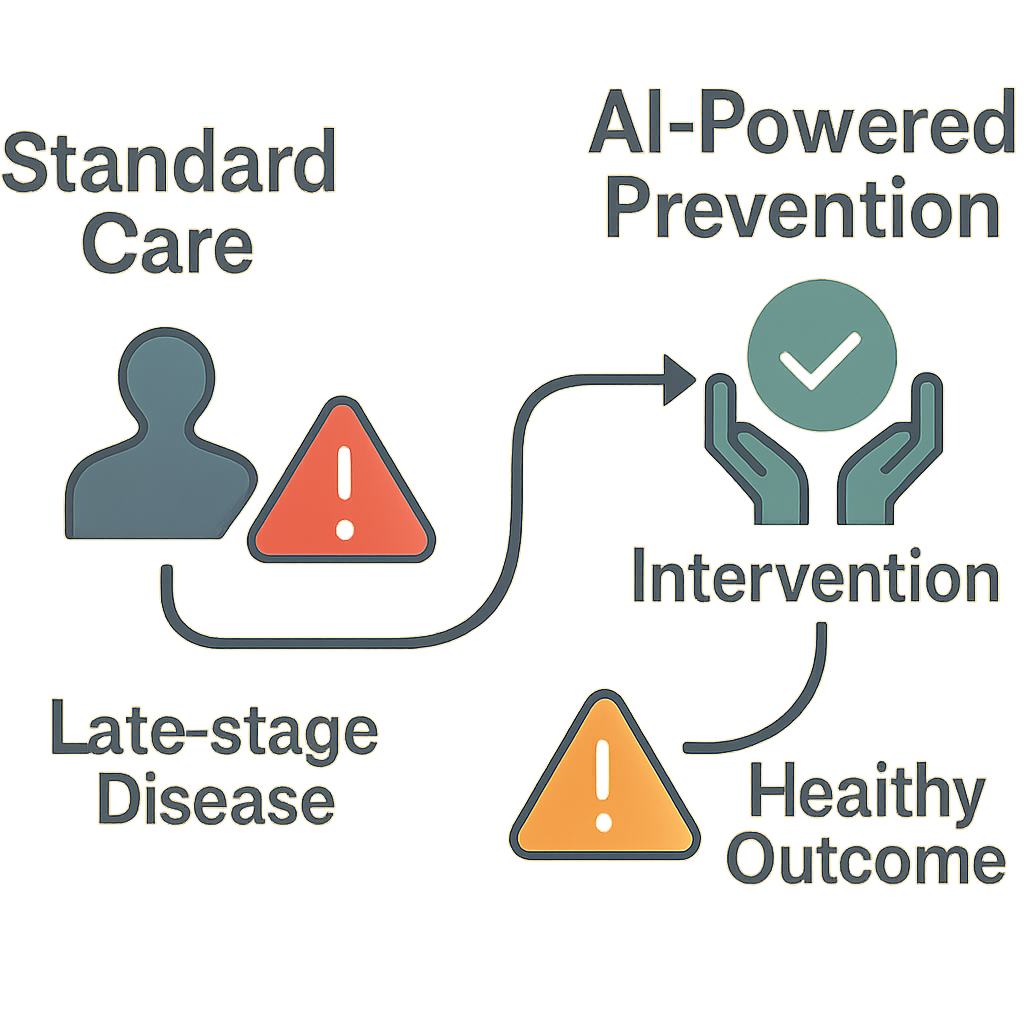
This is an incredible opportunity to change the way we play the game when it comes to addressing diseases. We will move from the reactive model of addressing disease once it is already symptomatic and has been diagnosed to the proactive model of Proactive Healthcare. For instance, if you find that you have a genetic predisposition to high cholesterol, you aren’t doomed by that discovery – rather, you now have the power to take advantage of the knowledge you’ve gained through your discovery.
This knowledge is one of the main ways that Deep Learning will benefit Genetic Research, and it is what will allow you to work with your physician to develop a personal prevention plan, based on a diet and/or exercise regimen, and possibly additional screenings. The Future of Artificial Intelligence in Healthcare will be more than just better treatment options for those who need them – it will be about reducing or eliminating many of the treatments so many need.
The technology will also have a profound impact on families with a hereditary component to their medical history. Families with members who have a hereditary disease will no longer have to worry about whether their children or grandchildren will develop the same disease.
The uncertainty associated with hereditary conditions will be replaced with the certainty of knowing the risks and steps you can take to prevent the disease from developing. The question will no longer be “Will I get this disease?” but rather “What can I do today to prevent me from getting this disease?” The use of Artificial Intelligence to provide an unparalleled view of our biological blueprints will give us the ability to choose our health destiny.
Supercharging the Lab: How AI Makes New Drug Discovery 10x Faster
Traditionally, discovering a new drug was similar to trying to find just one specific key to fit into a single, complicated lock, but you have a million keys on your keychain. For years, scientists tested many thousands of chemicals in a laboratory at a cost of billions of dollars, only to hope that one would be effective. The time-consuming, costly trial-and-error process is one reason it can take 10 or more years to develop a new medication.
Deep learning has greatly accelerated the process of identifying a drug target by applying “virtual screening” to identify millions of potential drug molecules. Scientists have identified a weakness or a drug target on the disease-causing cells, called a drug target (the lock). They then utilize an algorithm developed from AI for drug discovery to test digital simulations of how millions of potential drug molecules (the keys) may fit in the drug target.
This eliminates the need to manually test each compound, which could take a human their entire life to complete. Therefore, the researcher can quickly narrow down to the most promising candidates for further testing in a laboratory setting.
Deep learning has significantly improved genetic research by accelerating the process. Perhaps the greatest benefit of deep learning in genetic research is its impact on the thousands of rare genetic diseases that affect too many people to be researched affordably in traditional settings.
Although big data has enabled researchers to identify rare genetic targets, AI has enabled them to rapidly identify potential treatments for these diseases. By reducing a ten-year search to a matter of weeks, AI has given researchers the opportunity to offer families affected by a rare genetic disease new hope of finding a treatment.
The Double-Edged Sword: Protecting Your Most Personal Data
AI-based medicine has incredible promise — however, it depends on the most personal data possible: your genetic code. Unlike a stolen password, you cannot alter your DNA. Your genomic data not only provides insight into your current health; it also offers a glimpse into your future health risks, as well as those of your biological family members.
Because the sheer volume of genomic data creates significant barriers to analysis (in addition to the challenge of protecting it), these issues pose huge challenges for protecting genomic data.
In addition to the risks of a data breach through hacking, there are deep-rooted ethical implications in using AI in genetics. The main issue regarding AI in genetics is genetic discrimination. Genetic discrimination occurs when an individual is discriminated against due to their genetic information.
For example, if an insurance company increases your premiums because your genome shows a higher likelihood of developing heart disease, or if a potential employer passes you over for a job because your genome contains a marker for a neurological condition that may never occur in your lifetime. Neither of these examples is hypothetical; they are currently the subject of both legal and ethical debates.
Concerns about genetic discrimination in the context of AI in genetics have sparked a global dialogue on developing laws and regulations that will shape the future of artificial intelligence in healthcare. Scientists, ethicists, and legislators are actively engaged in establishing a framework that enables the continued advancement of genomics while protecting individuals’ rights. At this point, our society is having many fundamental discussions:
- Who owns my genomic data after I have had it sequenced?
- How is my genomic data being protected from hackers?
- Can insurance companies or employers use my genomic data against me?
Why AI Needs Genomic Data for Future Breakthroughs
The need for genomic data to enable future breakthroughs in Artificial Intelligence (AI) is now more urgent than ever as we enter a new era in which AI will fundamentally change how we understand Health & Disease. As such, the genomic data provides the foundational biological knowledge necessary to effectively train AI systems.
As AI algorithms are developed using genomic data, the sheer volume of genomic data enables the identification of patterns and correlations within large datasets, ultimately leading to the discovery of genetic variations associated with disease and to personalized medicine.
Additionally, the more genomic data the AI systems receive, the more precise and subtle their predictive capabilities become. Ultimately, this predictive capability is crucial to developing treatment plans tailored to each individual’s genetic profile, thereby maximizing the efficacy of therapeutics.
Additionally, using genomic data enables AI to examine the interaction between genetics and the environment. A significant part of this analysis is to recognize and understand how both are associated with one another.
The information gathered by analyzing this relationship is essential for predicting a person’s risk of contracting a disease and is an important step in developing a proactive health strategy. An AI model trained on genomic data from a large dataset can evaluate the relationships among multiple genetic markers and provide a basis for developing new preventive measures and treatment options.
The integration of genomic data with AI also offers significant benefits across many industries beyond medicine, including agriculture and drug development. Using genomic data to aid AI in analyzing it, researchers have been able to create plants/crops that grow stronger, produce more fruit or food, and resist certain diseases.
Additionally, AI has helped researchers identify new therapeutic targets for diseases at a much faster rate than before, thereby expediting the time it takes for a new drug to reach the public market.
Overall, genomic data is necessary for AI to make future discoveries and breakthroughs, as it provides the key insights needed to understand the complexity of human biology. With continued collection and analysis of genomic data, the collaboration between AI and genomics will be the driving force behind the next generation of medical and scientific developments, improving people’s health care and their overall quality of life globally.
The Future Is Here: Your Role in the Genomic Revolution
Not so long ago, reading your body’s genetic blueprint consisting of 3 billion letters was considered science fiction. However, you now recognize that the core issue in accessing this information is not that there isn’t enough of it, but rather that there is too much.
It will be clear to you that Artificial Intelligence is the means by which the hidden patterns embedded in genomic data are identified. This is the primary transition from mystery to manageable insight, and it will fundamentally alter the practice of medicine and transform healthcare from a reactive model to a proactive, personalized one.
The new generation of medicine being developed with AI technology will have a direct effect on your life as we know it. Therefore, begin developing knowledge of potential changes to our medical system now. First, simply discuss your family’s health history, which is the first type of genetic information.
Next, explore reliable, updated information regarding the NIH (National Institutes of Health), which has made many updates available. Your development of “genetic literacy” does not require a degree in science; however, if you wish to become involved in your health and wellness, you need to want to learn and develop the skills to do so.
Imagine the day when your check-ups move beyond the symptom-based model of health and become proactive, mapping out your path to health and wellness. Artificial Intelligence in healthcare is not intended to replace physicians; rather, its goal is to empower physicians with insights from your unique genetic code.
If you continue to be curious and ask questions, then you are no longer merely a patient; you are a partner in creating one of the most exciting chapters in human health and helping to create a world in which medicine is truly personal.




































Comments 1